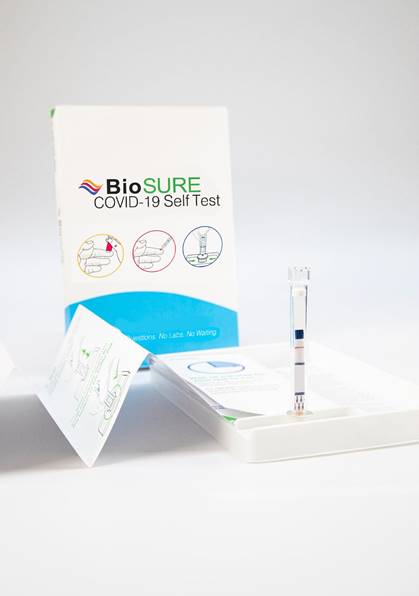
The BioSURE COVID-19 Antibody Self Test will be ready for mass production at the beginning of June, it is undergoing final validation to complete regulatory approvals. It will be available in the UK and global markets for corporate customers and directly to end-consumers from on-line platforms and retail pharmacies.
BioSure, experts in self-testing and developers of the BioSURE HIV Self-Test and Mologic Ltd. developer of advanced lateral flow and rapid diagnostic technologies, joined forces to produce this COVID-19 antibody self-test. Mologic’s co-founder, Professor Paul Davis, was one of the co-inventors of the Clearblue pregnancy test that was launched in 1988 as the world’s first commercial application of lateral flow technology.
By combining Mologic’s independently verified COVID-19 lateral flow test with BioSure’s market-leading HIV self-test design, the companies have created a self-test for COVID-19 that can be used by anyone, anywhere, without the need for any training. It is easy to use, requiring only a fraction of a drop of blood and gives the user their result in just 10 minutes. BioSure are now developing an app to record individual users’ results.
Nearly 3000 samples have been tested as part of the development and validation protocols for testing. Imperial College is using this information as part of their epidemiological mapping studies.
Mologic’s new diagnostic manufacturing facility in Bedfordshire, will be capable of producing 40millon tests a year. Building on a longstanding partnership, materials for all of Mologic’s COVID-19 diagnostics are being supplied to the Institute Pasteur de Dakar in Senegal who will manufacture 4 million tests for the African continent at their flagship facility diaTROPiX in Dakar.
Since March, alongside Liverpool School of Tropical Medicine and St George’s, University of London, leading laboratories across the world have partnered with Mologic to rapidly iterate, improve, and validate the Company’s COVID-19 diagnostic prototypes and independently assess performance.